What Best Describes the Bonding in a Carbon Dioxide Molecule
Why is carbon dioxide an element. Carbon dioxide does form hydrogen ions.
And compared to the ideal angle you would expect the actual angle between the nitrogen-chlorine bonds to be _____.
. Instead carbon dioxide is considered to be a compound. Total number of valence electrons in CO 2 1 x 4carbon 2 x 6oxygen 4 12 16. Carbon shares four of its electrons and each oxygen shares two of its electrons.
Carbon dioxide CO2 CID 280 - structure chemical names physical and chemical properties classification patents literature biological activities safety. Question 8 0303 MC What best describes the bonding in a carbon dioxide molecule. The more important one has two carbon oxygen double bonds It has two resonance structures.
The carbon dioxide molecule has two double bonds between the carbon and oxygen atoms. A structure of carbon dioxide molecule contains carbon atom at the center and each oxygen atom is placed on the left and right side of this carbon atom. It is MgCl2 because the total positive charge on Mg is two.
O It has two resonance structures. The more important one has a carbon oxygen triple bond O It has three resonance. One electron group means one lone pair one single bond one double bond or one triple bond.
The structural formula of a carbon dioxide molecule is written. A N smaller b Cl smaller c N equal-to d N larger e Cl larger. Electron dot structures or Lewis dot formula can be drawn if the molecular formula of the compound is known.
41 moles of methane molecules. Carbon shares four of its electrons and each oxygen shares two of its electrons. Each double bond is made up of one sigma and one pi bond so a carbon dioxide molecule contains two sigma and two pi bonds.
Let us draw the Lewis structure for carbon dioxide. Each oxygen atom shares a double. It is trigonal pyramidal because there are three bonded pairs and one lone pair around nitrogen.
Because of this chemical bond that is difficult to break carbon dioxide is not considered a mixture. Each electron pair is one bond. CH4g 2O2g CO2 g 2H2Og One mole of methane CH4 molecules reacts with two moles of oxygen molecules to form one mole of carbon dioxide molecules and two moles of water molecules.
What best describes the bonding in a carbon dioxide molecule. Carbon receives two electrons from each of the two oxygen atoms. Two bonds can be drawn as shown in the figure for CO 2 which accounts for four electrons 2 bond pairs.
The bond angle is 180 between both the carbon. We review their content and use your feedback to keep the quality high. Carbon and oxygen have two bonds each between their atoms.
The chemical symbol of the central atom is _____. Which statement best describes the structure of carbon dioxide. What best describes the bonding in a carbon dioxide molecule.
Carbon transfers two electrons to each of the two oxygen atoms. Carbon shares two of its electrons and each oxygen shares four of its electrons. These oxygen atoms are attached to carbon atom with double bonds on each side.
I Know its NOT Bond Formation resulsts in energy being used. One thing that we can understand by looking at the structure of CO 2 is that the carbon center of the molecule must be electrophilicAn electrophile electron-lover is a center that is electron poor and will be attracted to centers that are electron-rich. 18 Ar What phrase best describes the arrangement of these electron groups around the central carbon.
This is called a double bond. Which of these best describes why the carbon dioxide molecule is not polar. Who are the experts.
Carbon shares two of its electrons and each oxygen shares four of its electrons. Answer the questions in the table below about the shape of the carbon dioxide CO molecule li ilo How many electron groups are around the central carbon atom. Carbon receives two electrons from each of the two oxygen atoms.
Carbon shares four of its electrons and each oxygen shares two of its electrons. A molecule of the compound carbon dioxide contains one atom of the element carbon and two atoms of the element oxygen. The more important one has a carbon oxygen triple bond O It has three resonance structures.
What word or two-word phrase best describes the shape of the carbon dioxide CO2 molecule. See answer 1 Best Answer. The C-O bonds in carbon dioxide are polar and yet the dipole moment is zero because the 2 bond dipoles cancel each other.
Experts are tested by Chegg as specialists in their subject area. This answer is correct. Consider the nitrogen trichloride NCl3 molecule.
Draw single bonds between atoms. NH3 because nitrogen forms a single bond with each hydrogen atom. There are no ions present no or -charges in carbon dioxide gas because the electrons are shared not transferred from one atom to another.

Molecular Orbital Diagram Wikipedia The Free Encyclopedia Diagram Molecular Science Chemistry

Covalent Bond Definition Properties Examples Facts Britannica

Ch150 Chapter 4 Covalent Bonds And Molecular Compounds Chemistry

Covalent Bond Definition Types And Examples

Co2 Lewis Structure How To Draw The Dot Structure For Carbon Dioxide Youtube
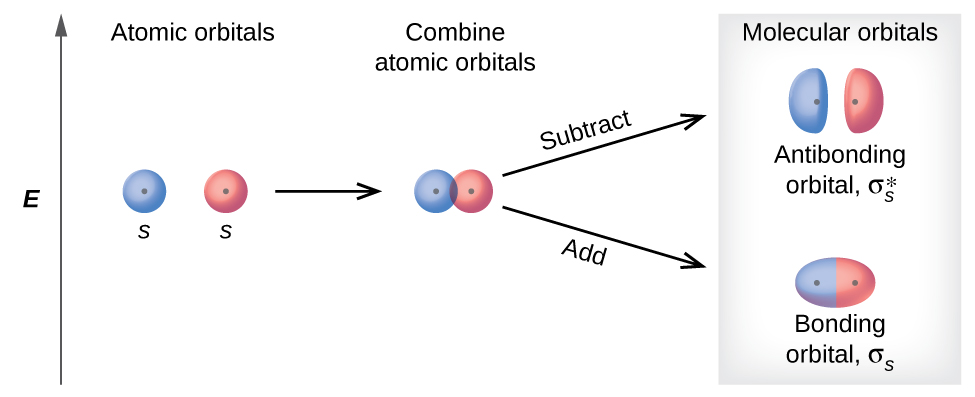
8 4 Molecular Orbital Theory Chemistry

Carbon Dioxide Molecule Co2 Lewis Dot Cross Electronic Diagram Covalent Bonds Ball Stick Space Filling 3d Models Boiling Point Melting Point Doc Brown S Chemistry Revision Notes

Which Statement Best Describes The Polarity Of Cf2i2 In 2022 Molecules Bond Best

Covalent Bond Definition Types And Examples

Molecules And Compounds Overview Atomic Structure Article Khan Academy
Atoms Isotopes Ions And Molecules Boundless Biology

Polar Covalent Bond An Overview Sciencedirect Topics

6 2 Comparing Ionic And Molecular Substances Chemistry Libretexts

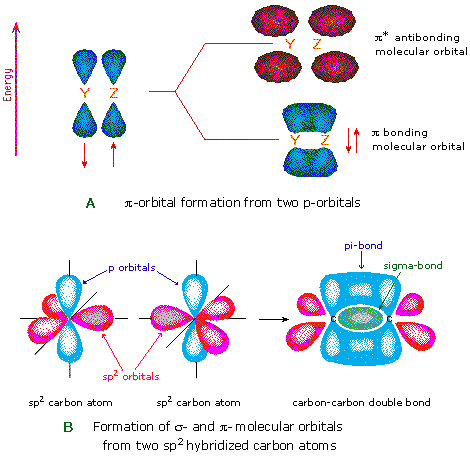
13 2 Molecular Orbitals For Ethene Organic Chemistry Ii

Type Of Bonds For Co2 Carbon Dioxide Youtube

The Shape Of The Hydrogen Peroxide Molecule In The Gas Phase Gas Molecules Tech Company Logos

Comments
Post a Comment